In 2021, the United States medical devices market was valued at 226 billion U.S. dollars. It is expected to increase to over 312 billion U.S. dollars by 2027, growing at a compound annual growth rate (CAGR) of 5.38%. This growing industry means competition is harder than ever.
With the right strategies, you can navigate this crowded space and have a successful medical device product launch checklist.
So, what should be included in the checklist to launch a medical device product?
A medical device product launch checklist should include regulatory compliance verification, market analysis, intellectual property protection, clinical trial data, and quality assurance testing. It should also cover marketing strategy development, stakeholder engagement, and others.
For a detailed guide, we’ll walk you through the essential steps for a successful launch, detailing everything you need to know. So, stick with us till the end.
What’s on this page:
Medical Device Product Launch Checklist: 8 Key Regulatory Considerations
Here are key medical device product launch plan that you should keep in mind:
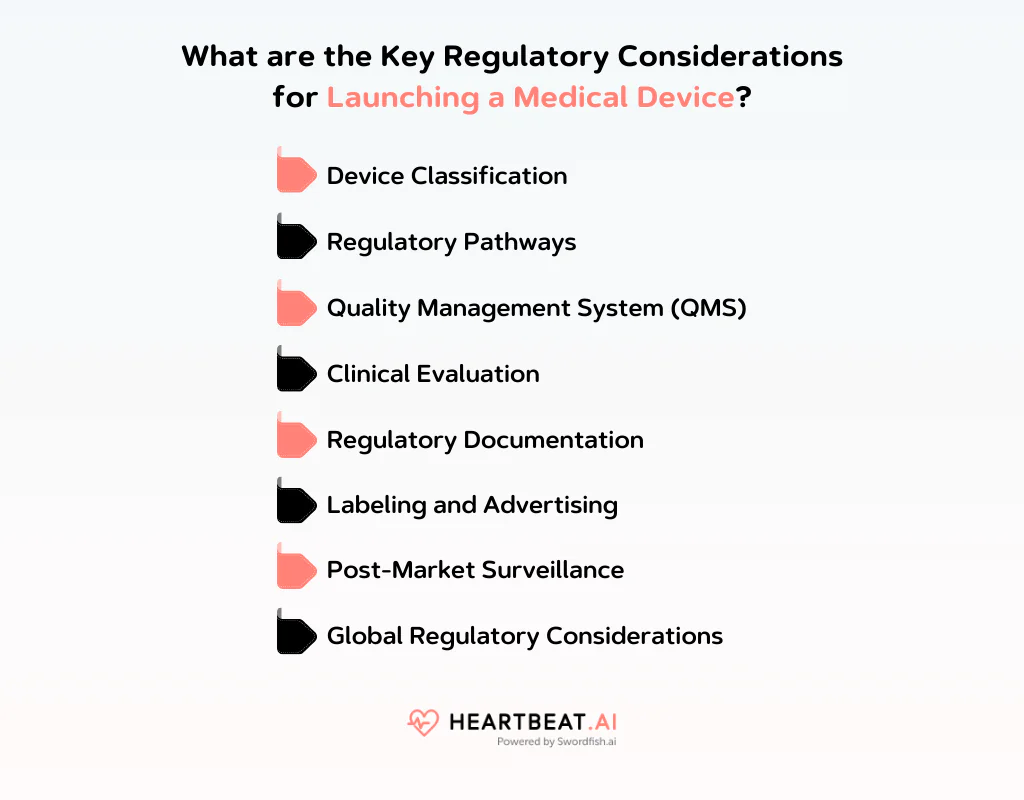
Device Classification
Medical devices are categorized by risk level, affecting the regulatory oversight required. Class I devices are low-risk, while Class III are high-risk, each subject to varying degrees of regulatory scrutiny.
Regulatory Pathways
Identify the appropriate regulatory pathway for your device based on its classification and intended use. This could range from general controls for low-risk devices to premarket approval for high-risk devices.
Quality Management System (QMS)
Implement a QMS compliant with standards like ISO 13485 to ensure your medical device is consistently produced to the highest quality standards.
Clinical Evaluation
Demonstrate your device’s safety and efficacy through clinical evaluation, which may include conducting trials to gather necessary data.
Regulatory Documentation
Prepare and submit a detailed regulatory dossier that includes technical documentation covering design, production, risk management, and clinical data to meet regulatory requirements.
Labeling and Advertising
Ensure all device labeling and advertising is accurate, avoiding false claims, and includes all necessary information such as usage instructions and safety warnings.
Post-Market Surveillance
Monitor your device’s performance after launch, reporting any adverse events to ensure ongoing safety and effectiveness.
Global Regulatory Considerations
Understand and comply with regulatory requirements in each market you plan to enter, as regulations can vary widely across different jurisdictions.
Why is a Detailed Medical Device Product Launch Checklist Important?
Creating a thorough medical device product launch checklist is essential. It helps ensure that every step in the process adheres to regulations, quality standards, and market needs. This detailed approach is important for several reasons:

Regulatory Compliance
Medical devices are subject to regulatory standards by bodies such as the FDA (U.S. Food and Drug Administration) and the EMA (European Medicines Agency). A detailed checklist ensures that every regulatory requirement is met, from pre-market approval to post-market surveillance, minimizing the risk of costly delays or legal issues.
Quality Assurance
The safety and effectiveness of medical devices directly impact patient health. A checklist helps maintain the highest quality standards throughout the development and launch process, covering design controls, manufacturing processes, and quality control tests. This careful examination helps avoid product recalls or harm to patients.
Market Readiness
Launching a medical device involves more than just meeting regulatory and quality standards; it also requires understanding the market. A detailed checklist ensures that market research, competitor analysis, and pricing strategies are completed, aligning the product with user needs and market demands.
Stakeholder Coordination
Medical device launches involve numerous stakeholders, including R&D teams, regulatory affairs, marketing, and sales departments. A detailed checklist facilitates coordination among these groups. This ensures that all parties are aligned with the launch timeline and objectives, and that communication is seamless.
Risk Management
Every stage of a medical device product launch involves potential risks, from design flaws to regulatory non-compliance. A checklist helps identify and reduce these risks early in the process, implementing corrective actions before they escalate into more significant problems.
Cost Control
Unexpected issues in the product launch process can lead to significant financial losses. A detailed checklist allows for better budgeting and resource allocation. This ensures that the project stays on track financially and that any potential cost overruns are addressed proactively.
Timeline Adherence
Delays in the product launch can result in lost market opportunities and increased costs. A checklist monitors progress against key milestones, ensuring that the project moves forward in a timely manner and that any delays are quickly addressed.
Post-Market Support
After a product is launched, the work is not over. A detailed checklist ensures that post-market surveillance, customer support, and ongoing regulatory reporting are in place to support the product throughout its lifecycle.
How to Launch a Medical Device Product?

Launching a medical device product involves navigating a complex process, from regulatory compliance to market entry and beyond. Here’s a concise overview of the key steps in the process.
Navigating Regulatory Requirements
Understanding and complying with regulations set by bodies like the FDA or EU’s MDR is crucial. The device’s classification affects the level of inspection it undergoes, involving testing, documentation, and possibly clinical trials, to ensure safety and efficacy.
Market Analysis and Strategy Development
A successful launch begins with thorough market research to understand target audiences, competitors, and trends. This informs a flexible strategic plan covering product positioning, pricing, and distribution.
Product Development and Testing
Development focuses on design iterations and usability testing to refine the product. Extensive testing ensures the device is effective, safe, and meets user needs.
Clinical Trials (if applicable)
For high-risk devices, clinical trials assess safety and efficacy in real-world settings. This complex step is essential for regulatory approval and market acceptance.
Manufacturing
Moving from prototype to mass production involves choosing manufacturing partners and setting up production lines. It also requires ensuring quality control and adhering to regulatory standards for packaging and labeling.
Marketing and Sales Strategy
Effective marketing educates the target market, including healthcare professionals and institutions. Strategies may involve medical conferences, digital campaigns, and direct engagement.
Launch and Post-Market Surveillance
The product launch aims for impact, followed by post-market surveillance to monitor performance, report adverse events, and refine the product based on feedback.
What Strategies Should be Used to Market a Medical Device Effectively?
Marketing a medical device effectively demands a strategic approach that addresses the unique challenges and regulations of the healthcare industry. Here are some tips for marketing checklist for product launch:

Understand Your Market and Audience
Comprehending your market and audience is crucial for customizing your medical device to meet specific needs and preferences. Analyze demographics, needs, and feedback to refine your product and messaging.
- Market Research: Begin with thorough market research to understand the healthcare sector, identify target demographics, and analyze competitors. Consider factors like market size, growth potential, and regulatory environment.
- Audience Segmentation: Segment your audience into categories such as healthcare professionals, patients, hospital procurement teams, and insurance companies. Customizing your message to each segment is crucial for relevance and impact.
Utilize Regulatory Approvals
Gaining regulatory approvals is important for launching a medical device, as it signifies compliance with critical safety and efficacy standards. Navigate the complex approval processes of entities like the FDA or EMA to access your target markets.
- Highlight Approvals: Use FDA approvals, CE marks, or any other regulatory endorsements as a cornerstone of your marketing strategy. Such approvals enhance credibility and can be a significant selling point.
- Educate Your Audience: Educate stakeholders about what these approvals mean and the rigorous testing and validation your product has undergone.
Emphasize Product Differentiation
Highlighting what sets your medical device apart from competitors is key to capturing market interest. Focus on unique features, benefits, and the specific problems your device solves to create a compelling value proposition.
- Unique Value Proposition (UVP): Clearly articulate what sets your device apart from competitors. This could be innovation, cost-effectiveness, improved patient outcomes, or ease of use.
- Case Studies and Clinical Trials: Present evidence from case studies and clinical trials to support claims about your device’s efficacy and safety.
Digital Marketing Strategies
Use digital platforms to reach and engage your target audience effectively. Utilize SEO, content marketing, social media, and targeted advertising to increase visibility and drive product interest.
- Website and SEO: Create a professional website with detailed product information, testimonials, and educational resources. Optimize search engines to increase visibility to those searching for solutions your device provides.
- Content Marketing: Produce valuable content such as blog posts, whitepapers, and videos that address common questions and concerns of your target audience.
- Social Media: Engage with your audience on platforms where they are active. LinkedIn can be particularly effective for B2B marketing in the medical field.
Engage with Healthcare Professionals
Building relationships with healthcare professionals is vital for gaining product endorsements and insights into clinical needs. Organize presentations, webinars, and face-to-face meetings to educate and receive feedback from these key stakeholders.
- Continuing Medical Education (CME): Offer CME courses related to your device, helping professionals stay informed and engaged with your product.
- Product Demonstrations and Trials: Facilitate hands-on experiences through demonstrations or trial periods, allowing healthcare professionals to assess the product’s impact firsthand.
Utilize Patient Advocacy and Testimonials
Incorporating patient advocacy and testimonials into your marketing strategy can significantly enhance trust and credibility. Share real-life success stories to demonstrate the impact of your medical device on patients’ lives.
- Patient Stories: Share real-life success stories and testimonials from patients who have benefited from your device. This humanizes your product and can significantly influence decision-making.
- Collaborate with Advocacy Groups: Partner with patient advocacy groups to raise awareness about your device, especially if it addresses specific conditions or patient groups.
Focus on Education and Thought Leadership
Establishing your brand as a thought leader through educational content helps build trust and authority in the medical community. Create and disseminate research papers, blog posts, and educational videos to share insights and innovations.
- Educational Workshops and Webinars: Host events that not only showcase your device but also provide valuable educational content for healthcare professionals.
- Publish Research: Regularly publish research findings in respected medical journals and present at industry conferences to establish your company as a thought leader in the field.
Build Strong Relationships with Distributors and Sales Reps
Promoting solid partnerships with distributors and sales representatives is crucial for expanding your product’s reach. Provide them with thorough training and support to ensure they are knowledgeable and motivated to sell your device effectively.
- Training and Support: Ensure your distributors and sales representatives are well-trained and have all the necessary information and support to effectively market your device.
- Incentive Programs: Implement incentive programs to motivate and reward high-performing sales personnel.
Monitor Feedback and Adapt
Actively monitoring customer and stakeholder feedback is essential for ongoing improvement and satisfaction. Use this input to make informed adjustments to your product, services, and marketing strategies, ensuring they meet the evolving needs and expectations of your market.
- Feedback Loops: Establish channels for receiving feedback from users and healthcare professionals. Use this feedback to make product improvements and refine your marketing approach.
- Market Adaptation: Stay adaptable to changes in the healthcare landscape, regulatory policies, and technological advancements.
Find the Right Healthcare Professional for Marketing Your Medical Device

Marketing your medical device in the healthcare sector is now simpler with Heartbeat AI. The platform helps you find healthcare professionals aligned with your product’s value proposition.
Here’s how Heartbeat empowers your marketing strategies:
Extensive Database Access
Heartbeat equips you with access to a broad and detailed database, packed with 11+ millions healthcare professional profiles. This resource is continuously updated and spans various specialties, ensuring you connect with the right audience.
Targeted Discovery with Prospector Tool
The Prospector tool is your insight generator, helping you identify healthcare professionals based on their interests and needs. It’s designed to refine your target list, making your outreach efforts both efficient and effective.
Instant Insights via Chrome Extension
For on-the-go access to professional profiles, the Chrome Extension integrates seamlessly into your browsing experience. It’s an instant research tool, giving you the ability to uncover professional details with a simple click, directly from your browser.
Enhance Your Leads with File Upload
If you’re starting with a pre-existing list of potential leads, the file upload feature enriches your list with comprehensive profiles and contact information, transforming basic data into a rich resource for your marketing strategies.
What are the Common Risks Associated With Launching a Medical Device?
Launching a medical device comes with various risks that need to be carefully managed to ensure safety, compliance, and successful market entry. Here are some common challenges:

Regulatory Compliance
Non-compliance with regulations and standards can result in delays or even bans on product launch. Ensuring that your medical device meets the requirements of FDA in the United States or the CE Mark in Europe is essential.
Safety Concerns
Safety is important in the medical device industry. Failing to identify and mitigate potential safety risks can lead to patient harm and legal liabilities. Thorough risk assessments and clinical trials are crucial.
Quality Control
Manufacturing defects or inconsistent product quality can harm both patients and your brand’s reputation. Implementing strict quality control measures is vital to minimize this risk.
Intellectual Property (IP) Issues
Protecting your device’s intellectual property through patents, trademarks, and copyrights is critical. Failure to do so may result in competitors copying your technology.
Market Competition
The medical device market is highly competitive. Entering a market saturated with similar products requires effective marketing strategies and differentiation to capture market share.
Reimbursement Challenges
Securing reimbursement from healthcare payers can be challenging. Without proper reimbursement, the commercial success of your device may be limited.
Clinical Trials and Data Collection
Conducting clinical trials to demonstrate safety and efficacy can be costly and time-consuming. Inadequate data collection or trial design can lead to regulatory setbacks.
Supply Chain Disruptions
Global events, natural disasters, or supply chain disruptions can affect the availability of components or materials needed for manufacturing.
Conclusion
In this guide, we’ve explored the essential strategies of the medical device product launch checklist.
To ensure a successful launch of your company’s medical device, it’s important that you follow these tips thoroughly. Each step, from understanding your market to ensuring regulatory compliance, plays a vital role in the launch process.
Remember, the success of your product depends on a clear, well-executed plan. By adhering to this straightforward approach, you’ll be better positioned to achieve your launch goals.
Frequently Asked Question
What is product release vs product launch?
Product release involves making the product available to the market. Product launch, on the other hand, refers to the marketing efforts to promote and introduce the product to potential customers.
What is the role of quality management systems (QMS) in medical device launch?
A QMS ensures your product is manufactured to the highest standards and complies with regulatory requirements. It covers all aspects of production, from design and development to manufacturing and post-market surveillance, ensuring consistency, safety, and effectiveness.
How to measure the success of medical device launch?
Success metrics might include sales figures, market penetration, customer feedback, product performance data, and regulatory compliance status. Set clear, measurable goals before launching and regularly review these metrics to assess progress and areas for improvement.