Are you aware that the United States has over 8,106 clinical trial managers, with an impressive 70.6% being women? This remarkable statistic underscores the vital role these professionals play in the world of healthcare and research.
And to engage with the licensed clinical trial managers, an accurate email list is vital, whether for networking, sharing breakthroughs, or promoting collaboration.
However, finding an up-to-date list can be challenging due to frequent industry changes and data privacy regulations. This is where Heartbeat AI comes into play.
Our platform offers advanced features like real-time data updates and comprehensive contact details, ensuring you access a reliable and current clinical trial managers email list. Keep reading to discover how Heartbeat AI can be your key to unlocking this valuable resource.
What’s on this page:
About HeartBeat’s Clinical Trial Managers Email Lists: What You Will Get
Heartbeat AI is a cutting-edge platform offering specialized email lists for clinical trial managers, customized to meet the precise needs of healthcare professionals. Here’s what you will gain from using our lists:
- Efficiency in Communication: With Heartbeat’s email list, you can smoothen your outreach efforts, ensuring your messages reach the right people.
- Market Insight: You can gain insights into the clinical trial landscape, helping you understand your target audience better.
- Time-Saving Resource: With Heartbeat AI’s email list, you can save your valuable time as the list is ready-to-use.
- Customizable Lists: You can customize outreach by selecting specific demographics, regions, or specialties within the clinical trial community.
- Reliability and Accuracy: You can trust in a list that is regularly updated, ensuring you have the most current and accurate contact information.
- Ease of Integration: Easily integrate the email list with your existing CRM or marketing tools, enhancing your workflow efficiency.
Types of Data Included in Clinical Trial Manager Email Lists
- Names of Clinical Trial Manager
- Location
- Contact Numbers (Work, Cell, Mobile)
- Email Address
- Specialization
- Years of Experience
- Sole Proprietor Status
- Licensed States
- License Number
- Fax
- Address
Accuracy & coverage of personal contact info were unrivaled by other platforms… this gives any healthcare recruiter a competitive advantage when engaging hard-to-reach healthcare providers.
Claim $500 of Free Data
Trusted by



By submitting this form, you are agreeing to Heartbeat’s
Benefits of Using the Valid Clinical Trial Managers Email Lists
Using a valid clinical trial managers mail list can significantly enhance your business and research endeavors in the medical and pharmaceutical fields. Here are the key benefits of utilizing such a list:
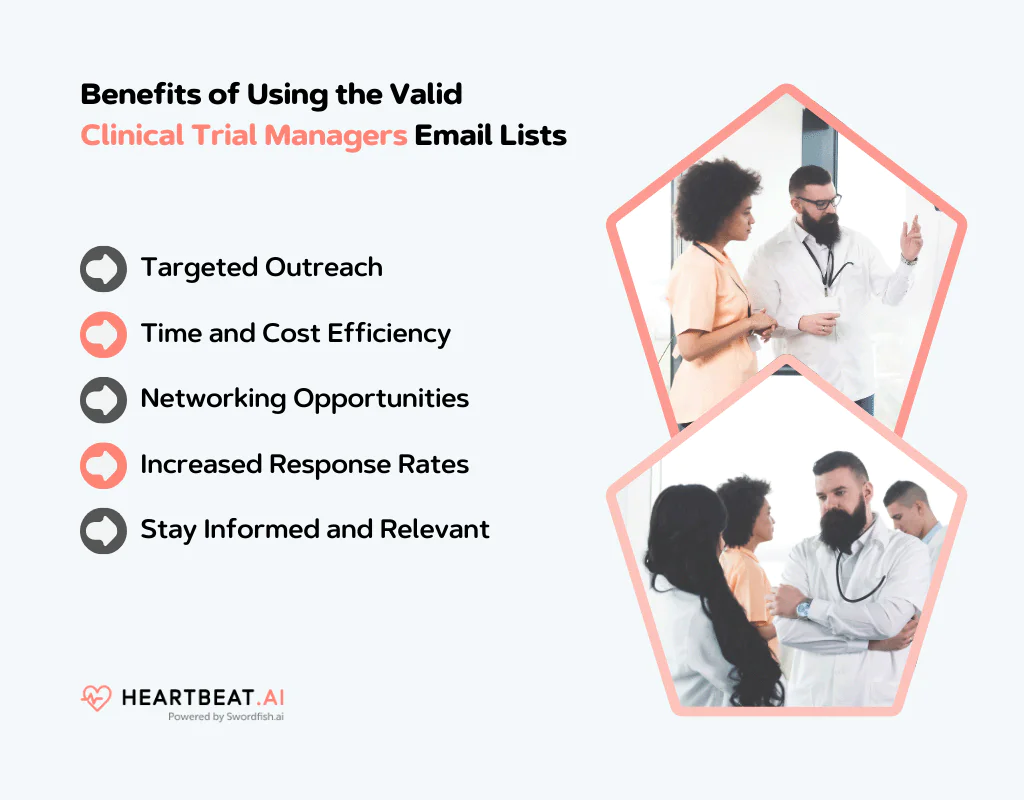
Targeted Outreach
You can reach out directly to clinical trial managers, who are key decision-makers in clinical studies. This precision targeting means your messages and offers are more likely to be relevant and well-received.
Time and Cost Efficiency
Instead of spending time and resources on broad, unfocused marketing, you concentrate your efforts where they count. This efficiency can lead to cost savings and better use of your time.
Networking Opportunities
It’s important to build a network in clinical research. With access to a relevant email list, you’re one step closer to forming valuable connections with industry professionals.
Increased Response Rates
Since you’re reaching out to a targeted audience, the response rate to your emails is likely to be higher compared to generic marketing campaigns.
Stay Informed and Relevant
Regular communication with clinical trial managers keeps you informed about the latest trends and needs in the field. This allows you to customize your products or services accordingly.
Who Are You Likely to See in Our Clinical Trial Manager Mailing List by Category?
In our clinical trial manager email directory, you’ll discover various primary care clinical trial manager, each with their unique and captivating skills. Here is the list of clinical trial manager of different categories:
| Clinical Trial Manager Categories |
| Clinical Research Manager |
| Clinical Operations Manager |
| Clinical Data Manager |
| Clinical Trial Coordinator |
Available Clinical Trial Manager in Our Clinical Trial Manager Email Database by States
If you’re seeking a comprehensive list of clinical trial manager by cities, we have compiled a detailed table for your reference. This table includes a clinical trial manager email database located throughout various states across the United States.
How Do We Compile Our Data to Create a Clinical Trial Manager Email List Database?
When we create a clinical trial manager email list database at Heartbeat AI, our process is thorough and focused on quality. Here’s how we do it:
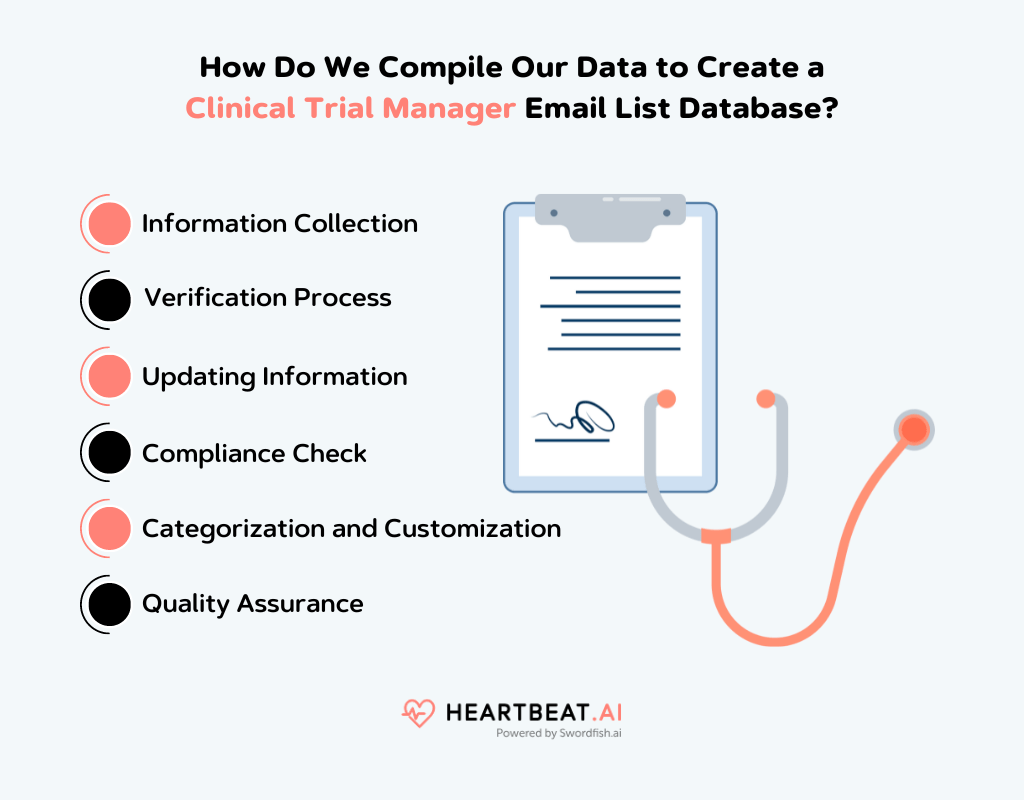
Information Collection
We start by gathering information from our 200 reliable sources. This includes medical directories, healthcare forums, professional networking sites, and industry-specific conferences. We ensure all our sources are credible and up-to-date.
Verification Process
Once we have this data, we go through a rigorous verification process. We check each piece of information for accuracy. This includes confirming the email addresses, job titles, and affiliations of the clinical trial managers. We use both automated tools and manual checks to ensure accuracy.
Updating Information
The healthcare industry is dynamic, with frequent changes in job roles and contacts. We regularly update our database to reflect these changes. This ensures that the information you receive is current and relevant.
Compliance Check
It’s important to us that our database complies with legal standards like GDPR and HIPAA. We make sure that all the data we collect and provide is in line with these regulations, respecting the privacy and rights of the individuals listed.
Categorization and Customization
We categorize the data based on various factors like geographical location, area of expertise, and experience level. This makes it easier for you to find exactly what you need. We also offer customization options if you have specific requirements.
Quality Assurance
Before we finalize the database, our quality assurance team conducts a thorough review. They check for any errors or inconsistencies to ensure that the database you receive is of the highest quality.
Why Choose Heartbeat.ai’s Clinical Trial Manager Mailing List?
Selecting Heartbeat.ai’s clinical trial manager mailing list can greatly improve your communication efforts in the healthcare sector. Here’s why we believe it’s a great choice:

Comprehensive Data Coverage
At Heartbeat.ai, we have a vast database that includes more than 11 million healthcare professionals in the United States. This extensive dataset covers a wide range of healthcare providers, including clinical trial managers, doctors, nurses, dentists, pharmacists, and more.
High Accuracy and Speed
We take pride in the accuracy of our data, with an impressive 84% accuracy rate for cell phone numbers and 92% for personal email addresses. This precision, combined with the speed of accessing this information, makes your outreach efforts more efficient and productive.
Heartbeat Prospector
Our Heartbeat Prospector tool is a savior. It allows you to find clinical trial managers by their specialties. This tool ensures that you connect with the right professionals quickly and accurately.
Chrome Extension for Ease of Use
Our Chrome Extension enhances your ability to discover contact information. It seamlessly integrates with platforms such as LinkedIn and Facebook, granting you the capability to effortlessly locate contact details.
Integration and Automation Support
For more advanced needs, Heartbeat.ai offers support for integration and automation. This includes features like bulk file enrichment and API access, which can be particularly valuable for larger-scale and sophisticated outreach campaigns.
Flexible Pricing Plans
We understand that different users have varying needs, so we offer a range of pricing plans. Whether you’re an individual headhunter or part of a large agency or enterprise, our flexible pricing options ensure that you can select a plan that aligns with your budget and requirements.
What to Consider When Getting an Email List of Clinical Trial Managers?
When you’re considering getting an email list of clinical trial managers, there are several important factors to keep in mind:
Relevance
Always ensure the list includes managers who are relevant to your field or area of interest. It’s not just about having a long list, but about having the right contacts.
Accuracy
You should check how often the list is updated. Outdated lists can lead to a high rate of undelivered emails, which is a waste of your time and resources.
Source
Always know where the list comes from. Is it compiled through reputable means? Avoid lists that might have been gathered through questionable methods, as this can harm your credibility.
Privacy Compliance
You must make sure the mail list of clinical trial managers complies with privacy laws like GDPR or HIPAA. Using a list that violates these laws can get you into legal trouble.
Customization
Always check if you can get a list that’s customized to your specific needs. A generic list might have many irrelevant contacts.
Cost
You should consider the cost of the list and weigh it against the potential benefits. Don’t overspend on a list that doesn’t offer good value for your needs.
Now, Who Can Use the Clinical Trial Manager Email List?
The clinical trial manager mail List can be used by a variety of professionals and organizations in the healthcare and pharmaceutical sectors. Here are some of the key groups who might find this list particularly useful:

Pharmaceutical Companies
They can use the list to connect with clinical trial managers for discussions about new drugs, treatments, or to seek partnerships for clinical trials.
Biotechnology Firms
Biotechnology firms, especially those involved in developing new medical technologies or treatments, can use the list to collaborate on research and clinical trials.
Medical Device Manufacturers
For companies that produce medical devices, the list can be instrumental in finding the right experts to conduct or oversee trials related to their products.
Research Institutions and Universities
Academic and research institutions might use the list to find collaborators for clinical research or to share findings and insights.
Contract Research Organizations (CROs)
CROs often require contacts in clinical trials to outsource certain research activities or to partner for comprehensive trial management services.
Healthcare Consulting Firms
These firms can use the list to offer their services, ranging from regulatory compliance advice to trial design and management consulting.
How to Utilize Clinical Trial Manager Email Lists Effectively
To effectively utilize clinical trial manager mailing lists, follow these straightforward steps:

Identify Your Objective
Before you start, be clear about what you want to achieve. Are you looking to inform about a new product, seek collaboration, or gather feedback? Your goal will shape your approach.
Segment the List
Not all clinical trial managers will be interested in the same content. Segment your list based on criteria like their specialization, geographic location, or past interactions. This helps customize your messages to be more relevant.
Craft Your Message
Write emails that are clear, concise, and to the point. Focus on how your message benefits the clinical trial managers. Avoid using complex jargon; instead, use language that’s easy to understand.
Personalize
Personalization goes beyond just adding the recipient’s name. Customize your content to address the specific needs or interests of the clinical trial managers. Show that you understand their challenges and can offer solutions.
Compliance and Permissions
Ensure you comply with email marketing laws and regulations, like GDPR or CAN-SPAM Act. Only send emails to those who have consented to receive them.
Test and Optimize
Send out different versions of your email to small segments of your list to see which performs better. Look at open rates, click-through rates, and responses to measure effectiveness.
Conclusion
Throughout this guide, we have looked into the essential aspects of a valid clinical trial managers email list. We’ve discussed the challenges that come with ensuring the accuracy and relevance of such a list, as well as the significant benefits it can bring to the healthcare industry.
Having access to a quality email list can smoothen communication, promote collaborations, and speed up vital research initiatives.
For those seeking a dependable source of clinical trial managers mail lists, we recommend giving Heartbeat AI a try. As a trusted provider in this field, Heartbeat AI can provide you with the essential contact information you need to connect with key figures in the world of clinical trials.
Frequently Asked Questions
Are there any guidelines for effective email outreach to clinical trial managers?
Yes, crafting personalized and relevant emails, respecting opt-in/opt-out preferences, and providing value in your messages are essential.
What are the benefits of using a list of clinical trial manager with email addresses for networking?
Networking with professionals in the field can lead to collaborations, knowledge sharing, and potential business opportunities.
Can I use the clinical trial manager with email addresses for purposes other than marketing, such as research or surveys?
Yes, you can use the email list for various purposes, as long as they align with the list’s intended use and privacy regulations.